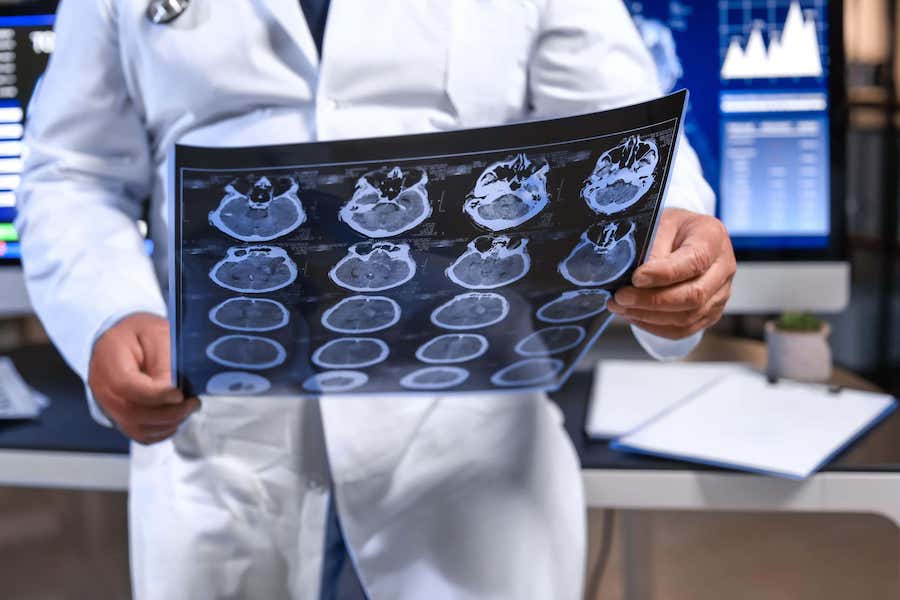
New drug found to slow Alzheimer’s hailed as turning point
Donanemab has been found to slow down the disease in a clinical trial
A new drug has been hailed as a “turning point in the fight against Alzheimer’s” after it was found to slow the progression of the disease.
Donanemab was found to slow “clinical decline” by up to 35%, meaning that people with the disease could still go about performing day-to-day tasks including shopping, housekeeping, managing finances and taking medication.
Alzheimer’s Research UK said that “we’re entering a new era where Alzheimer’s disease could become treatable”.
The Alzheimer’s Society said that treatments like donanemab could one day mean the condition could be likened to other long-term ailments such as asthma or diabetes.
Today’s full results support what we heard about donanemab in May – the drug is able to slow down the progression of Alzheimer’s disease by more than 20%.
This is truly a turning point in the fight against Alzheimer’s disease. #donanemab #AAIC23 pic.twitter.com/0UsTnQda6G
— Alzheimer’s Society (@alzheimerssoc) July 17, 2023
The charity said new treatments including donanemab – which works by removing a protein called amyloid that builds up in the brains of people with Alzheimer’s – heralds a “new era” for Alzheimer’s disease treatments.
The health spending watchdog in England is already assessing whether the drug can be used in the NHS.
It comes as scientists published the final results of the trial examining the safety and efficacy of the drug, manufactured by Eli Lilly and Company.
Researchers examined almost 1,800 people with early-stage Alzheimer’s, half of them received a monthly infusion of donanemab and the other half were given a dummy drug, also known as a placebo, over 18 months.
The study, published in the Journal of the American Medical Association and presented to the Alzheimer’s Association International Conference in Amsterdam, concluded that after 76 weeks of treatment, donanemab was able to slow clinical decline by 35.1% in people with early Alzheimer’s whose brain scans showed low or medium levels of a protein called tau.
When the results were combined for people who had different levels of this protein, there was a 22.3% slowing in disease progression.
Some 47% of people taking the drug who had early-stage disease and low or medium levels of tau were found to stall the disease for a year.
Eli Lilly and Company said some people taking the drug would be able to finish the course of treatment in six months once their amyloid plaque cleared.
It said treatment with donanemab reduced amyloid plaque on average by 84% at 18 months, compared with a 1% decrease among people in the study who were taking the placebo drug.
But researchers did find that among a small number of people in the study there were some serious side effects such as brain swelling.
The company said it is ready to work with health regulators in the UK as well as the NHS and Government on the “appropriate regulatory next steps”.
The National Institute for health and Care Excellence (Nice) said that it has already started its appraisal work on the drug.
The success of the phase 3 trial of Alzheimer’s drug #donanemab, has been confirmed today at Alzheimer’s Conference #AAIC2023.
Thanks to decades of research, we’re entering a new era where Alzheimer’s disease could become treatable.
Our full comment 👇 https://t.co/bknp9ut8cq pic.twitter.com/fg0UUnSKZH
— AlzheimersResearchUK (@AlzResearchUK) July 17, 2023
Mike Colley, who has been on the global trial of donanemab for two years, told BBC News: “I am one of the luckiest people you will ever meet, just for this.”
Mr Colley, whose memory and ability to process information have still been impacted by the illness, added: “I seem to get more confident every day and I am sure this is going to be successful.
“I am sure they will get all the rubbish off the top of my brain and I will be back to normality. I am very confident about that.”
His son Mark Colley added: “I never thought I would see my dad just so full of life again. Now we have hope, a few years ago we didn’t and that is just an incredible difference.”
Before Nice gives the drug the green light for NHS use, it needs to be approved by the medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA).
The results come after another drug – lecanemab – was found to reduce memory decline among patients with early-stage disease.
Commenting on the results, Dr Richard Oakley, associate director of research and innovation at Alzheimer’s Society, said: “This is truly a turning point in the fight against Alzheimer’s and science is proving that it is possible to slow down the disease.
“Treatments like donanemab are the first steps towards a future where Alzheimer’s disease could be considered a long-term condition alongside diabetes or asthma – people may have to live with it, but they could have treatments that allow them to effectively manage their symptoms and continue to live fulfilled lives.”
“Just as we’ve seen a transformation in cancer treatment in recent decades, we’re really hopeful we’re on the same path for dementia.”
Dr Oakley also told BBC Breakfast: “In the last 12 months we’ve had two trials, one announced last November and one this afternoon, and these trials show (these drugs) remove a protein called amyloid from the brain really effectively, and that seems to slow down the progression of the disease.
“And it keeps people being able to do things like drive a car, manage finances, talk about current affairs, recognise family members for longer. And that is so important.
“So we really believe this is the beginning of a whole new era for Alzheimer’s disease.”
Dr Susan Kohlhaas, executive director of research and partnerships at Alzheimer’s Research UK, said: “Today’s announcement marks another milestone.
“Thanks to decades of research, the outlook for dementia and its impact on people and society is finally changing, and we’re entering a new era where Alzheimer’s disease could become treatable.
“As a potential first-generation treatment, donanemab’s effects are modest. But these results provide further confirmation that removing amyloid from the brain can change the course of Alzheimer’s, and may help people affected by this devastating disease if they’re treated at the right time.”
Sir John Hardy, professor of neuroscience and group leader at the UK Dementia Research Institute, UCL, added: “The successful outcome of the Eli Lilly’s anti-amyloid antibody donanemab is great news for Alzheimer’s disease and confirms the positive and similar outcome for Eisai’s lecanemab trial late last year.
“The results are very similar, and that in itself is reassuring.
“Disease progression is slowed about 30%, but it too has occasionally serious complications which require monitoring.”
Former prime minister David Cameron, who is president of Alzheimer’s Research UK, said it was a real “breakthrough”.
He told BBC Radio 4’s PM programme it was a “moment to now put more resources into further research, to try and speed up the process of getting the drugs that will really make a difference”.
“Let’s fund the research, let’s do the trials, let’s get these drugs going, let’s get them simpler and more effective. I am hopeful our system can deliver this,” he said.
The Press Association
Latest posts by The Press Association (see all)
- BBC to air two-part Call The Midwife Christmas special - December 23, 2024
- 6 mind sports to exercise your brain and keep you sharp - December 20, 2024
- Quiz: What classic Christmas food or drink are you? - December 20, 2024
- Leftover turkey and watercress pie - December 20, 2024
- Catherine and William choose family shot for Christmas card photograph - December 19, 2024